Batteries
What are Electrical Batteries?
- Electrical batteries are electrochemical devices that convert stored chemical energy into electrical energy through redox reactions.
- They consist of one or more electrochemical cells, each containing:
- Anode (negative electrode): Where oxidation occurs, releasing electrons
- Cathode (positive electrode): Where reduction occurs, accepting electrons
- Electrolyte: Medium that allows ion flow between electrodes while preventing electron flow
- Separator: Physical barrier that prevents direct contact between electrodes while allowing ion transport
- When a load is connected, electrons flow from anode to cathode through the external circuit, while ions move through the electrolyte to maintain charge balance.
- This creates a sustained electrical current until the chemical reactants are depleted.
Electron Flow in Cells
- Traditional notation for flow of current for discharging is from +ve terminal to -ve terminal
- This is called Galvanic Flow
- In Electron Flow notation, the actual direction of flow of electrons is shown
- During discharging, electrons flow into the +ve terminal
- During charging, electrons flow into the -ve terminal
- While charging, potential difference (voltage) across terminals increases, as more and more electrons reach the -ve terminal
- The +ve terminal becomes more positive as fewer and fewer electrons are present
- The -ve terminal becomes more negative as more and more electrons are present
- While discharging, this process is reversed, as voltage begins to drop
- As more electrons reach the +ve terminal, it becomes lesser and lesser positively charged
- At the -ve terminal, as more and more electrons leave, it also becomes less and less negatively charged
- Potential Difference (Voltage) as a result reduces
To view Electron Flow Theory in action during Charging and Discharging, Click here
Lead Acid Chemistry
Construction
Core Components:
- Positive Electrode: Lead dioxide (
) on lead grid - Negative Electrode: Spongy lead (
) on lead grid - Electrolyte: Sulfuric acid solution (
+ , specific gravity 1.25-1.28) - Separator: Microporous material AGM, gel, or liquid-flooded, see The Differences Between AGM, GEL and FLOODED Batteries
- Container: Polypropylene or ABS plastic housing
- Terminals: Lead alloy posts for external connections
Cell Configuration:
- Voltage: 2.0V per cell
- 12V Battery: 6 cells connected in series
- Grid Structure: Lead-antimony or lead-calcium alloy grids support active material
Working Principle
How Lead Acid Batteries Work:
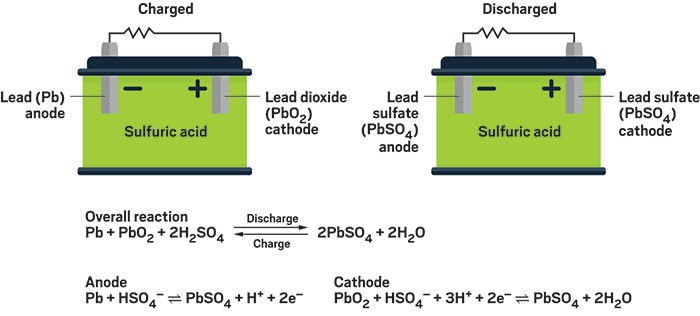
When Discharging (Starting Your Car or Powering Equipment):
- The battery contains lead plates - one made of pure spongy lead (
)(negative) and one coated with lead dioxide ( ) (positive) - Both plates sit in sulfuric acid, which acts like a chemical soup
- When you turn the key, both lead plates react with the sulfuric acid
- This reaction converts both plates into the same material: lead sulfate (a white, chalky substance)
- During this conversion, electrons are released and flow through your car's electrical system
- The sulfuric acid gets weaker as it's used up in the reaction, and water is produced
- Eventually, both plates become coated with lead sulfate and the acid becomes mostly water - the battery is "dead"
When Charging (Alternator or Battery Charger):
- The charger forces electricity back into the battery, reversing everything that happened
- The lead sulfate on both plates converts back to their original materials
- Negative plate becomes pure lead again, positive plate becomes lead dioxide again
- The weak acid and water recombine to form strong sulfuric acid
- The battery is restored to its original state and ready to work again
Simple Analogy: Imagine two different colored sponges (lead plates) sitting in colored water (sulfuric acid). When you squeeze them (discharge), they both turn the same color and the water becomes clear. When you stretch them back out (charge), they return to their original colors and the water becomes colored again.
Why It Works: The beauty of lead acid batteries is that this chemical conversion is reversible hundreds of times, though eventually the plates wear out and can't convert properly anymore.
How to Tell if It's Charged:
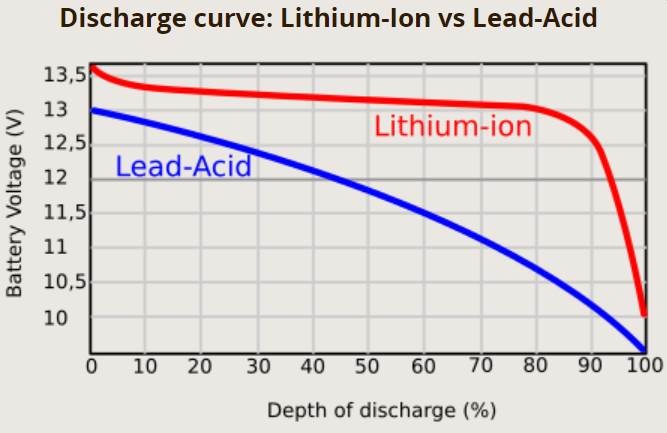
- Voltage Test: A fully charged 12V battery reads about 12.6V, while a dead one reads around 10.5V
- Acid Strength: The stronger the sulfuric acid (higher specific gravity), the more charged the battery
- Physical Signs: Some batteries have a built-in "magic eye" indicator that changes color based on charge level
Lead Acid Advantages:
- Low cost: Economical initial investment
- Robust: Tolerates overcharge and deep discharge better
- Recyclable: 95%+ material recovery rate
- Proven technology: Mature, well-understood chemistry
- High surge current: Excellent for starting applications
Li ion Chemistry
Construction
Core Components:
- Cathode: Lithium metal oxide (e.g.,
, , ) - Anode: Graphite (
) with intercalated lithium - Electrolyte: Organic liquid (lithium salt in organic solvent like
in EC/DMC) - Separator: Microporous polymer membrane (PE/PP)
- Current Collectors: Aluminum foil (cathode), Copper foil (anode)
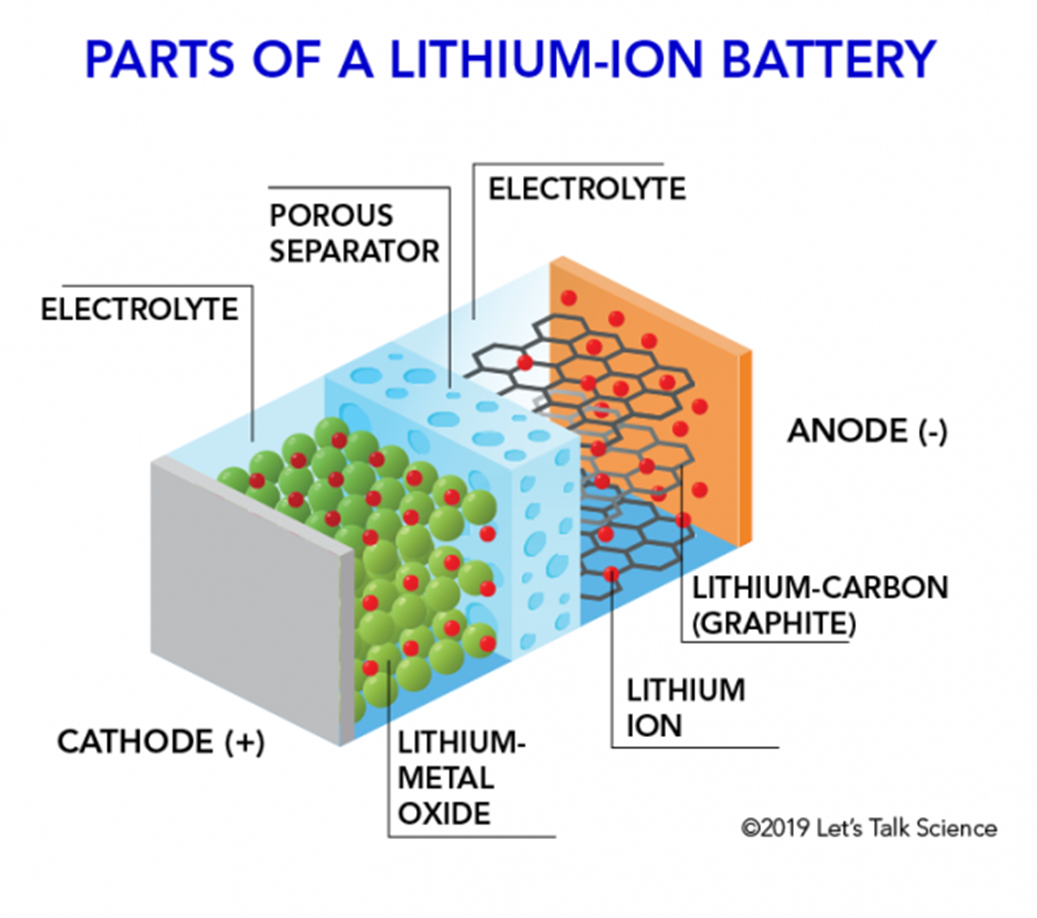
Physical Structure:
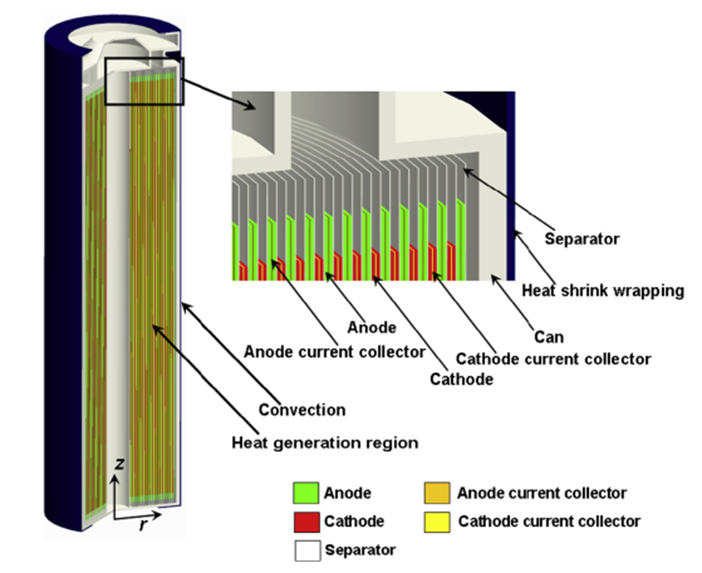
- Cylindrical: Rolled electrode sheets (18650, 21700 cells)
- Prismatic: Flat electrode stacks in rectangular housing
- Pouch: Flexible aluminum-polymer laminate
Working Principle
How Li-ion Batteries Work:
Think of a Li-ion battery like a bus shuttle system:
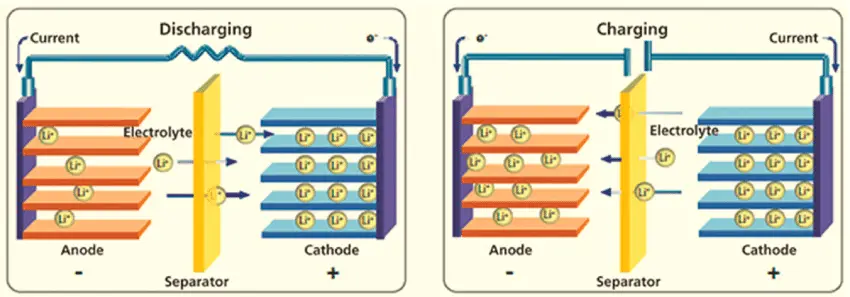
When Discharging (Powering Your Device):
- Lithium ions (tiny charged particles) are stored in the graphite anode, like passengers waiting at a station
- When you use the battery, these lithium ions travel through the liquid electrolyte (like a highway) to reach the cathode
- At the same time, electrons flow through the external wire (your device's circuit) from the negative to positive terminal
- This electron flow is what powers your phone, laptop, or electric car
- The process continues until most lithium ions have moved to the cathode side
When Charging:
- You plug in the charger, which applies electrical pressure to push everything backwards
- Lithium ions travel back from the cathode to the graphite anode through the electrolyte
- The graphite anode acts like a parking garage, storing the lithium ions between its layers
- Once fully charged, all the lithium ions are back in the anode, ready to make the journey again
- The battery is now "reset" and ready to power your device again
Simple Analogy: Imagine a water wheel where water (lithium ions) flows from a high reservoir (anode) to a low reservoir (cathode), turning the wheel on its way down. Charging is like pumping the water back uphill to refill the high reservoir.
Li-ion Advantages:
- High energy density: Compact and lightweight
- No memory effect: Can be partially charged/discharged
- Low self-discharge: Retains charge well during storage
- Fast charging: Can accept high charge rates
- Long cycle life: Thousands of charge/discharge cycles
Comparison between Lead Acid & Lithium ion Chemistry
| Parameter | Lead Acid | Lithium Ion |
|---|---|---|
| Energy Density | 30-50 Wh/kg | 150-250 Wh/kg |
| Cycle Life | 200-300 cycles | 500-2000+ cycles |
| Operating Temperature | -20°C to +50°C | -20°C to +60°C |
| Memory Effect | None | None |
| Depth of Discharge | 50% (deep cycle) | 80-100% |
| Initial Cost | Low ($50-150/kWh) | High ($200-600/kWh) |
| Maintenance | Regular (water topping) | Minimal |
| Safety | Hydrogen gas risk | Thermal runaway risk |
| Environmental Impact | Lead toxicity concern | Mining impact, recyclable |
| Weight | Heavy (11-18 kg/kWh) | Light (2.5-6 kg/kWh) |
| Voltage per Cell | 2.0V | 3.6-3.7V |
| Efficiency | 70-85% | 95-98% |
| Lifespan | 3-5 years | 8-15 years |
| Charge Estimation | Fairly Easy (Voltage - % Charge Linear relation) | More Complex (using advanced algorithms and models in BMS) |
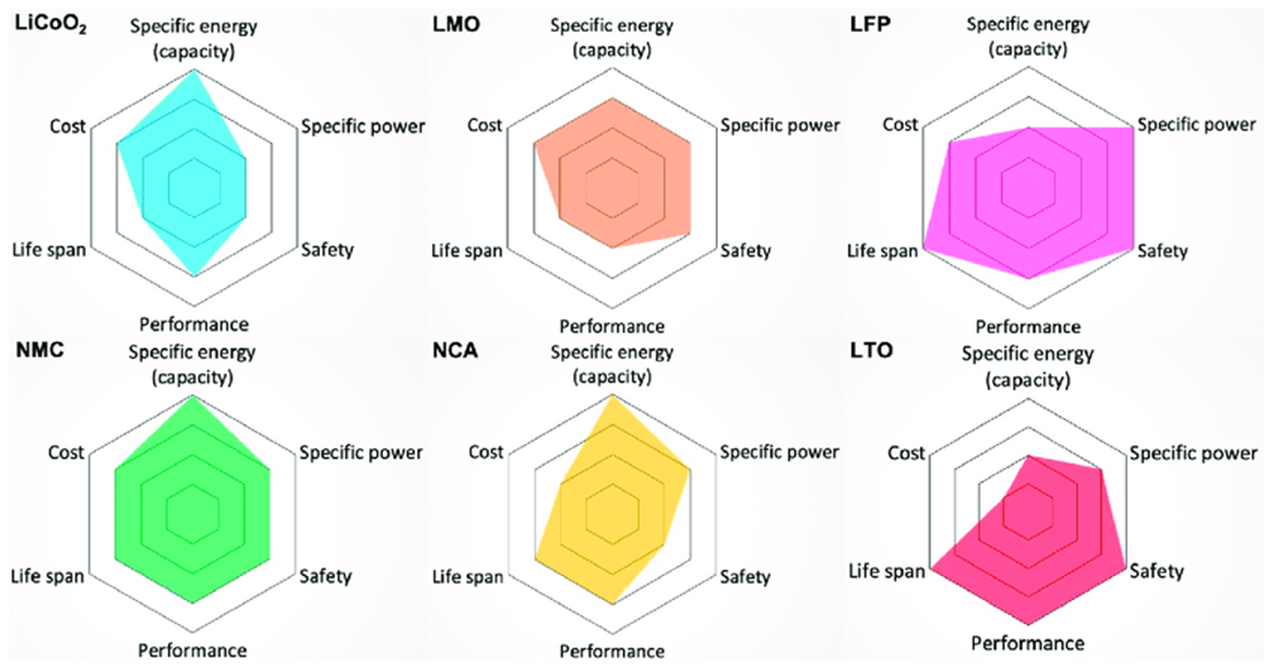
Lithium Ion Chemistry Comparison
Please slide to the right to view all Chemistry Types in the comparison table
| Chemistry | LCO | LTO | NMC | NMA | NMO | LMO | LFP |
|---|---|---|---|---|---|---|---|
| Full Name | Lithium Cobalt Oxide | Lithium Titanate | Nickel Manganese Cobalt | Nickel Manganese Aluminum | Nickel Manganese Oxide | Lithium Manganese Oxide | Lithium Iron Phosphate |
| Chemical Formula | |||||||
| Nominal Voltage | 3.6V | 2.4V | 3.6-3.7V | 3.6V | 4.7V | 3.8V | 3.2V |
| Energy Density | 150-200 Wh/kg | 70-80 Wh/kg | 150-220 Wh/kg | 200-260 Wh/kg | 140-150 Wh/kg | 100-150 Wh/kg | 90-120 Wh/kg |
| Cycle Life | 500-1000 | 10,000+ | 1000-2000 | 500-1000 | 1000+ | 300-700 | 2000-5000 |
| Operating Temp | -20 to +60°C | -30 to +55°C | -30 to +60°C | -20 to +60°C | -20 to +60°C | -20 to +50°C | -20 to +60°C |
| Safety | Moderate | Excellent | Good | Good | Good | Good | Excellent |
| Cost | High | High | Medium | Medium | Medium | Low | Medium |
| Toxicity | High (Cobalt) | Low | Medium | Low | Low | Low | Very Low |
| Self-Discharge | 2-3%/month | 5%/month | 3-5%/month | 3-5%/month | 3-5%/month | 4-8%/month | 3-5%/month |
| Fast Charging | Good | Excellent | Good | Good | Limited | Fair | Good |
| Applications | Smartphones, laptops, tablets | Electric buses, grid storage, fast charging | Electric vehicles, power tools, ESS | Tesla vehicles, high-energy EVs | High voltage applications, telecom | Medical devices, power tools | EV (China), home storage, marine |
Key Considerations
- Energy vs Power Trade-off: Higher energy density chemistries typically have lower power density and vice versa.
- Safety vs Performance: Safer chemistries like LFP and LTO often sacrifice some energy density for improved thermal stability.
- Cost vs Performance: More advanced chemistries with better performance typically come at higher costs due to material composition and manufacturing complexity.
- Cycle Life vs Energy: Chemistries optimized for longevity (LTO, LFP) may have lower energy density but significantly longer operational life.
Click here to view a sample quiz worksheet,
Check if your understanding of battery concepts are correct!
Click here to continue to Battery Pack Design & Calculations


